HEPES
| |
| Names | |
|---|---|
| Preferred IUPAC name
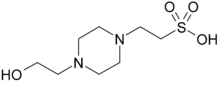
2-[4-(2-Hydroxyethyl)piperazin-1-yl]ethane-1-sulfonic acid | |
| Other names
HEPES
| |
| Identifiers | |
3D model (JSmol)
|
|
| 883043 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.098 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H18N2O4S | |
| Molar mass | 238.3012 g/mol |
| Appearance | white crystalline powder |
| Density | Not applicable |
| Melting point | >234-238°C (453-457K) |
| 40 g/100 ml (20°C) | |
| Acidity (pKa) | 3 (pKa1), 7.5 (pKa2)[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Eye Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a zwitterionic sulfonic acid buffering agent; one of the twenty Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration (produced by aerobic respiration) when compared to bicarbonate buffers, which are also commonly used in cell culture. [2] Lepe-Zuniga et al. reported an unwanted photochemical process wherein HEPES catalyzes a reaction with riboflavin when exposed to ambient light to produce hydrogen peroxide.[3][4] This is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep solutions containing both HEPES and riboflavin in darkness as much as possible to prevent oxidation.
HEPES has the following characteristics:
- pKa1 (25 °C) = 3
- pKa2 (25 °C) = 7.5
- Useful pH range = 2.5 to 3.5 or 6.8 to 8.2
HEPES has negligible metal ion binding,[5] making it a good choice as a buffer for enzymes which might be inhibited by metal chelation.
See also
[edit]References
[edit]- ^ Johnson MA, Seifert S, Petrache HI, Kimble-Hill AC (2014). "Phase Coexistence in Single-Lipid Membranes Induced by Buffering Agents". Langmuir. 30 (33): 9880–9885. doi:10.1021/la5018938. PMC 4148158.
- ^ Baicu SC, Taylor MJ (2002). "Acid-base buffering in organ preservation solutions as a function of temperature: new parameters for comparing buffer capacity and efficiency". Cryobiology. 45 (1): 33–48. doi:10.1016/S0011-2240(02)00104-9. PMID 12445548.
- ^ Lepe-Zuniga JL, Zigler JS, Gery I (October 1987). "Toxicity of light-exposed Hepes media". Journal of Immunological Methods. 103 (1): 145. doi:10.1016/0022-1759(87)90253-5. PMID 3655381.
- ^ Zigler JS, Lepe-Zuniga JL, Vistica B, Gery I (May 1985). "Analysis of the cytotoxic effects of light-exposed HEPES-containing culture medium". In Vitro Cellular & Developmental Biology. 21 (5): 282–7. doi:10.1007/BF02620943. PMID 4019356. S2CID 6557697.
- ^ "Hopax Fine Chemicals - Biological buffers and their interactions with metal ions".

