Talk:Plum pudding model
| This It is of interest to the following WikiProjects: | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
|
This page has archives. Sections older than 90 days may be automatically archived by ClueBot III when more than 4 sections are present. | |
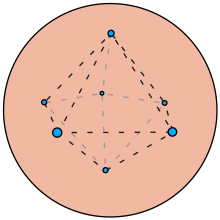
My diagram
[edit]@Ajrocke and Johnjbarton: I drew this diagram of the plum pudding model based on a 1905 diagram by JJ Thomson. The electrons are arranged in a pentagonal dipyramid, equidistant from the center. Is this a good diagram or misleading? Kurzon (talk) 19:15, 28 April 2024 (UTC)
- As a re-drawing of a historical diagram I think it is fine. I will tweak the caption. Johnjbarton (talk) 15:24, 29 April 2024 (UTC)
- Should all the electrons be the same distance from the center of the sphere? I wonder if the peaks of the pyramids should be a different distance. Kurzon (talk) 08:48, 12 July 2024 (UTC)
- The diagram looks like the ones in Thomson's paper. Johnjbarton (talk) 00:37, 13 July 2024 (UTC)
- Have you seen one that shows multi-shell electrons? I have heard of the "Thomson problem" in mathematics but all the diagrams I find constrain the electrons to the surface of the sphere. Kurzon (talk) 06:52, 13 July 2024 (UTC)
- I have not see such a diagram. Thomson's analysis of the multi-ring case was very limited. Above 6 electrons he finds that stability requires adding electrons in the center of the atom. Above 9 electrons the number of electrons in the center increase until at 15 he needs 15 in the center. (Recall that as more electrons are added the positive sphere also increases in strength). The electrons in the center then repel, leading to an inner ring. By this analysis he gets multi-rings. But he cannot do stability calculations on multiple rings, he's just guessing at this point.
- Bohr later said the equivalent of "he's making stuff up". Pais notes that Thomson generally considered physical models to be stories useful for generating ideas rather than the basis of quantitative analysis. (I can something about this to the article if you think it useful). And note that the great success of Bohr's later shell model follows the idea of rings of special stability. Johnjbarton (talk) 16:25, 13 July 2024 (UTC)
- Nobody has tried a computer simulation? Kurzon (talk) 18:20, 13 July 2024 (UTC)
- Perhaps if we expand the section on the Thomson problem one will come up. Johnjbarton (talk) 02:25, 14 July 2024 (UTC)
- Nobody has tried a computer simulation? Kurzon (talk) 18:20, 13 July 2024 (UTC)
- Have you seen one that shows multi-shell electrons? I have heard of the "Thomson problem" in mathematics but all the diagrams I find constrain the electrons to the surface of the sphere. Kurzon (talk) 06:52, 13 July 2024 (UTC)
- The diagram looks like the ones in Thomson's paper. Johnjbarton (talk) 00:37, 13 July 2024 (UTC)
- Should all the electrons be the same distance from the center of the sphere? I wonder if the peaks of the pyramids should be a different distance. Kurzon (talk) 08:48, 12 July 2024 (UTC)
- It looks fine to me.Ajrocke (talk) 17:56, 30 April 2024 (UTC)
Overview is too long and detailed.
[edit]The Overview is too dense. I'm not even sure why it exists. Seems like what we need is "Background". The intro should be the overview. Johnjbarton (talk) 03:00, 27 June 2024 (UTC)
- Ok I moved content out of Overview, and deleted some of it. In its place I added a Background section with four ingredients essential for the Thomson story: atomic model, electrons, radiation, and spectral lines. Thomson uses electrons to build a model of the atom, radiation to probe matter in support of his model, but ultimately fails to describe spectral lines. Johnjbarton (talk) 18:48, 27 June 2024 (UTC)
Need a section on the experimental evidence.
[edit]Thomson and his colleague Crowther published work on the scattering of beta particles by metal foils that they used to support Thomson's model. This work should be discussed. Johnjbarton (talk) 03:09, 27 June 2024 (UTC)
Development
[edit]Unfortunately at least some of content of the Development section is wrong, and now I suspect it all. It appears to be a synopsis of self-selected contributions of Thomson by date, created by reading the original papers. It's a good example of why Wikipedia prefers secondary sources.
For example the 1905 lecture was an overview of previous work, esp. a 1903 paper where the magnetic analogy was introduced based on previous work by Alfred Marshall Mayer. Johnjbarton (talk) 21:48, 27 June 2024 (UTC)
- In my experience working on many history projects on Wikipedia, secondary sources are often unreliable. They often present a distorted summarization of what came before. That's why I use both. Kurzon (talk) 22:04, 27 June 2024 (UTC)
- Well I agree that many pages use web sites as if they were secondary sources or sensationalized pop-science articles that aren't historical analysis. Johnjbarton (talk) 00:50, 28 June 2024 (UTC)
- Also secondary sources might contradict each other and we end up having to pick and choose and interpret anyway . Kurzon (talk) 22:17, 27 June 2024 (UTC)
Lead sentence is inappropriate.
[edit]The lead sentence is currently
- The plum pudding model is an obsolete scientific model of the atom.
This incorrectly makes "obsolete" the most significant characteristic of the Thomson model. That does not, for example, distinguish it among other articles on atomic models:
- Bohr atom – Atomic model introduced by Niels Bohr in 1913
- Cubical atom – Early atomic model
- Rutherford model – First atomic structure proposal to include a nucleus
or models without their own page, including Joseph Larmor's Solar System model (1897), Jean Perrin's model (1901), Hantaro Nagaoka's Saturnian model (1904), Arthur Haas's quantum model (1910), and John William Nicholson's nuclear quantum model (1912). The distinguishing feature of the Thomson model was the first use of internal structure. In addition the concept of "obsolete" is not a major point of discussion in the article as an aspect of the model, making "obsolete" a secondary point.
I changed the first sentence to one that matches the subject, but @Kurzon changed it back. I disagree and want a sentence that describes this model more clearly. Johnjbarton (talk) 17:00, 28 June 2024 (UTC)
- The point you want to make is just a few sentences further. It's ok. Kurzon (talk) 17:01, 28 June 2024 (UTC)
- I disagree. If a later sentence is adequate for the most important characteristic of the model (being the first with internal structure), then it certainly is adequate for the least important characteristic (being obsolete). Johnjbarton (talk) 17:53, 28 June 2024 (UTC)
- I like it because it signals to students "You don't have to know any of this for the exam. You can take a nap instead of reading this if you want.". Kurzon (talk) 21:14, 28 June 2024 (UTC)
- That's not a Wikipedia goal. Plus any student who can't figure this out is probably napping already. Johnjbarton (talk) 00:52, 29 June 2024 (UTC)
- I like it because it signals to students "You don't have to know any of this for the exam. You can take a nap instead of reading this if you want.". Kurzon (talk) 21:14, 28 June 2024 (UTC)
- I disagree. If a later sentence is adequate for the most important characteristic of the model (being the first with internal structure), then it certainly is adequate for the least important characteristic (being obsolete). Johnjbarton (talk) 17:53, 28 June 2024 (UTC)
- Alternatives include:
- The plum pudding model was the first modern scientific model of the atom.
- Per
- Kragh, Helge. "Before Bohr: Theories of atomic structure 1850-1913." RePoSS: Research Publications on Science Studies 10 (2010). https://css.au.dk/fileadmin/reposs/reposs-010.pdf
- "The atomic model developed by the famous Cavendish physicist Joseph John Thomson in the early years of the twentieth century can with some justification be called the first modern model of the atom".
- Kragh, Helge. "Before Bohr: Theories of atomic structure 1850-1913." RePoSS: Research Publications on Science Studies 10 (2010). https://css.au.dk/fileadmin/reposs/reposs-010.pdf
- or
- The plum pudding model was the first scientific model of the internal structure of the atom.
- Per
- "J. J. Thomson's plum-pudding atomic model: The making of a scientific myth" Giora Hon, Bernard R. Goldstein 06 September 2013 https://doi.org/10.1002/andp.201300732
- "What distinguishes Thomson's theory is his assignment of a specific inner structure to the atom as well as a set of dynamical assumptions."
- "J. J. Thomson's plum-pudding atomic model: The making of a scientific myth" Giora Hon, Bernard R. Goldstein 06 September 2013 https://doi.org/10.1002/andp.201300732
- Johnjbarton (talk) 18:27, 28 June 2024 (UTC)
- I have incorporated these references and an additional secondary history ref in a new section called "Significance". Johnjbarton (talk) 03:50, 14 July 2024 (UTC)
- @Kurzon Please stop changing the lead sentence without discussion. It is inappropriate per sources and personally rude in my opinion. Johnjbarton (talk) 16:13, 21 July 2024 (UTC)
- I have incorporated these references and an additional secondary history ref in a new section called "Significance". Johnjbarton (talk) 03:50, 14 July 2024 (UTC)
Proposal for new organization of Development section.
[edit]Currently the Development section has chronological year-named subsections corresponding to some of Thomson publications or lectures. Several secondary references discuss this work as having two phases, one culminating in Thomson's 1904 paper and related lectures and a second phase triggered by his discovery that the number of electrons per atom is similar to the atomic weight ratio to hydrogen in 1906. Here is what A. Pais writes in Inward Bound:
- The period 1897-1913 consists of two distinct parts. In the first, it was believed that the number of electrons in the atom is large. In the second, it was realized that this number is of the order of the atomic number. This change was wrought by Thomson, in 1906.
I propose an organization like:
- Development (or Models?)
- Polyelectron atomic model
- Magnet analog, mechanical stability, comparison to periodic chart and chemistry.
- Revised model
- Discovery of the number of electrons per atom, consequences for the model.
- Beta scattering theory and experiment.
- First efforts to directly test atomic model. Initial success.
- Polyelectron atomic model
Johnjbarton (talk) 16:23, 29 June 2024 (UTC)
- Eventually maybe. In writing history a good approach is to begin with a chronological approach, because timing is everything in history. Then as the information matures, it could perhaps be reorganized into something else. First, add the stuff you want to add with the chronological system. Kurzon (talk) 17:03, 29 June 2024 (UTC)
Removing incorrect reference.
[edit]The reference
- Alviar-Agnew, Marissa; Agnew, Henry (4 April 2016). "4.3: The Nuclear Atom". Introductory Chemistry. LibreTexts. Retrieved 9 February 2021.
is very short. It claims
- "The electron was discovered by J. J. Thomson in 1897. The existence of protons was also known, as was the fact that atoms were neutral in charge. Since the intact atom had no net charge and the electron and proton had opposite charges, the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom."
The proton was not properly identified until at least 1917. Thomson's models had no protons. The reference appears in support of "as was the fact that atoms were neutral in charge". However this is not needed in the article because the reason Thomson needed positive charge was stability, not neutrality. Johnjbarton (talk) 23:23, 30 June 2024 (UTC)
- Also the ref says:
- "In Thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge like blueberries stuck into a muffin."
- which is complete baloney. Johnjbarton (talk) 23:25, 30 June 2024 (UTC)
J. Arnold Crowther
[edit]One of the main protagonists in the atomic theory of 1905-1910 is James Arnold Crowther. Given so many dubiously notable biographies on Wikipedia I was surprised to discover nothing about him here. In addition to his publications on beta scattering, consider published books:
- Crowther, James Arnold. Ions, electrons, and ionizing radiations. Longmans, Green, 1924.
- "Many have owed their first acquainance with modern physics to this excellent intermediate text, which reached its eigth and final edition in 1949, the year before Crowther's death." Heilbron, 1968, footnote on page 296.
- Crowther, James Arnold. A manual of Physics. H. Frowde, 1919.
- Crowther, James Arnold. Molecular Physics. J. & A. Churchill, 1923.
- Crowther, James Arnold. The life and discoveries of Michael Faraday. Society for promoting Christian knowledge, 1920.
His obitutary:
- Whiddington, R. "Prof. JA Crowther." Nature 165.4202 (1950): 750-751.
Johnjbarton (talk) 18:02, 6 July 2024 (UTC)
- I created a page for James Arnold Crowther.
 Done Johnjbarton (talk) 16:06, 12 July 2024 (UTC)
Done Johnjbarton (talk) 16:06, 12 July 2024 (UTC)
Thomson problem
[edit]Has anyone ever done a computer simulation of a Thomson atom with 100 or so atoms, arranged in shells like Thomson imagined? The Thomson problem solutions I found online constrain the electrons to the surface of the sphere. Kurzon (talk) 16:45, 11 July 2024 (UTC)
- Thomson's 1906 paper discusses analytic stability of his shells in detail. Johnjbarton (talk) 16:59, 11 July 2024 (UTC)
- I am looking for a diagram. Kurzon (talk) 08:44, 12 July 2024 (UTC)
Dalton's was the first
[edit]@Johnjbarton: I don't want to be pedantic, but Dalton's model of the atom was the first. It had atomic weights, which is not much but something. Kurzon (talk) 21:44, 13 July 2024 (UTC)
- I have previously discussed this issue under Lead_sentence_is_inappropriate. Johnjbarton (talk) 03:48, 14 July 2024 (UTC)
- You're good at physics, not so much prose. Kurzon (talk) 13:49, 22 July 2024 (UTC)
- How about this as the first sentence:
- The plum pudding model was the first scientific model of the atom with internal structure.
- This simple declarative sentence notes the historical significance of the topic. Dalton's model did not have internal structure. Johnjbarton (talk) 18:01, 22 July 2024 (UTC)
- How about this as the first sentence:
- You're good at physics, not so much prose. Kurzon (talk) 13:49, 22 July 2024 (UTC)
How is this image incorrectly labelled?
[edit]Explain. Kurzon (talk) 18:50, 25 July 2024 (UTC)
- The image that was added to the page did not have the electron labeled. Johnjbarton (talk) 20:42, 25 July 2024 (UTC)
- Well once again there is no edit summary but this time the image is complete. Johnjbarton (talk) 21:05, 25 July 2024 (UTC)
- B-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Physical sciences
- B-Class level-5 vital articles
- Wikipedia level-5 vital articles in Physical sciences
- B-Class vital articles in Physical sciences
- B-Class physics articles
- Mid-importance physics articles
- B-Class physics articles of Mid-importance
- B-Class physics history articles
- Physics history articles
- B-Class history of science articles
- Mid-importance history of science articles
- WikiProject History of Science articles



